The hierarchical Linnean system provides different levels for grouping organisms. One of these is provided by Orders, which break Classes into groups of related Families. The addition in FishBase 2000 of a new table for the 62 Orders of fishes thus provides users a convenient access to their related Families, and thence to Genera and Species sharing broadly similar features. Further, the broad outlines of the classification of fishes are now largely agreed upon by taxonomists (see e.g., Nelson 1994; Helfman et al. 1997; and Eschmeyer 1998). The next step is thus to give a time dimension to this consensus classification, as this added dimension can help answer questions about the timing of major evolutionary events and the spread of diversity at the various levels of classification. It also provides a link into the fossil record. See also Box 3 for a discussion of phylogeny.
The ORDERS table makes use of recent work by one of us (D. Preikshot), wherein trees depicting taxonomic affinities are combined with dated fossils to derive, using cluster analysis, a time scale for the trees’ branching pattern. The affinities considered here are those implied in the classification of Eschmeyer (1998), whose tree is very similar to that depicted on the frontispiece of Nelson (1994). Corresponding information from the fossil record was extracted from Caroll (1988), Colbert and Morales (1991), Forey et al. (1993), Forey and Janvier (1993), Helfman et al. (1997), Patterson (1977), Pough et al. (1989), Shirai (1996) and others.
Fig. 5, which can also be called from within the ORDERS table, illustrates the tree thus obtained. This ‘tree of fish life’ combines temporal and relational information on fish groups in a manner that is readily accessible. One feature of this tree is that it allows straightforward identification of the ‘Sister group’ of any Order, as well as defining the time since two Orders last shared an ancestor. Because cluster analysis was used to generate the tree, linkages which occur above the level of Order suggest temporal and phylogenetic relationships based on the common ancestor information. Thus, the tree also provides a hypothesis-generating platform for investigating fish relationships at or above the level of Order. Lastly, the tree, or parts thereof, can be expanded to the level of Family, Genus and Species given the input of relevant data.
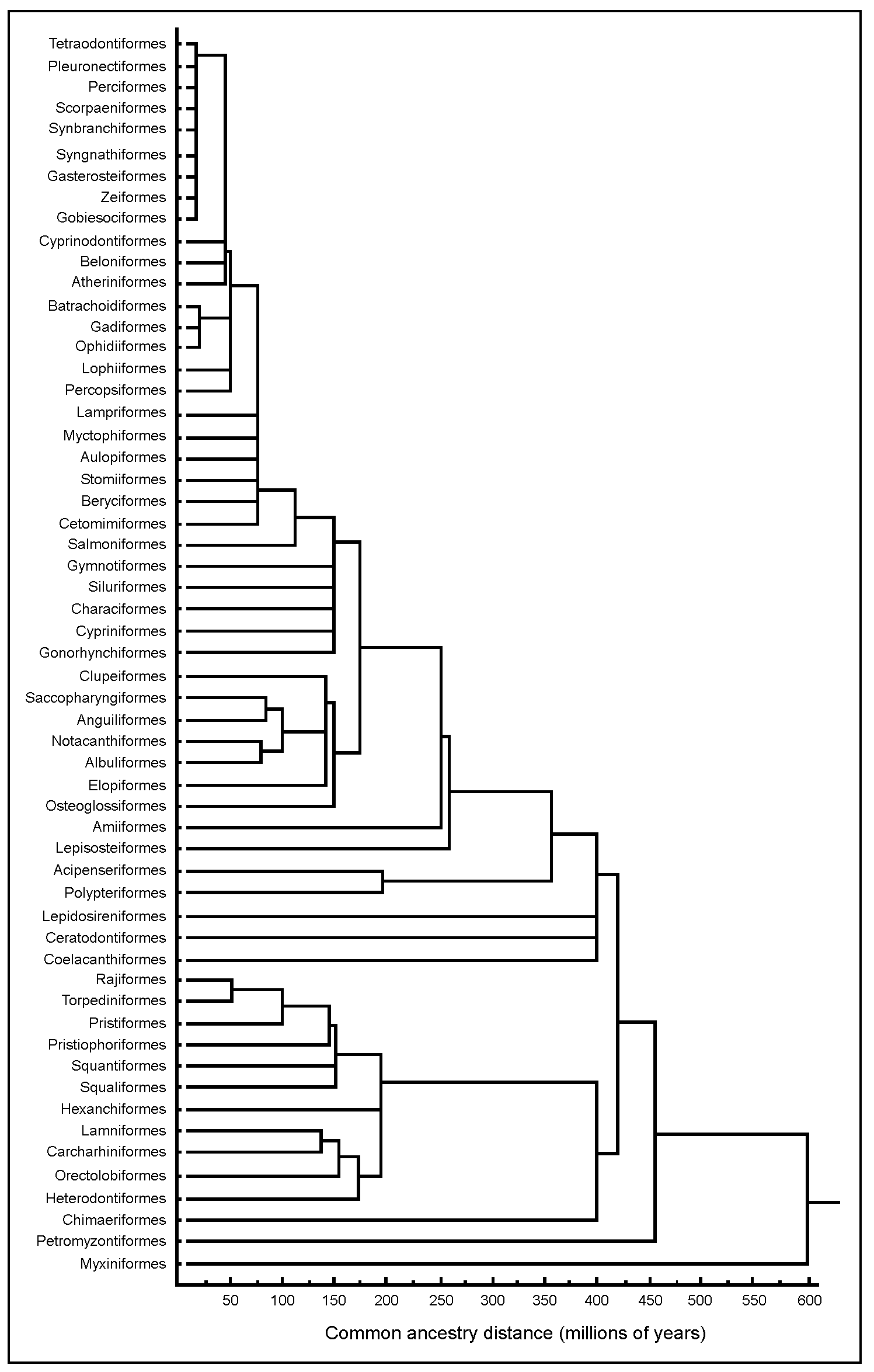
Fig. 5. Cluster analysis of extant of fishes as determined by evidence of common ancestry or by the appearance of fossil forms.
The term ‘fish’ includes hagfishes, lampreys, chondrichthyans (sharks, rays, chimaeras), actinopterygians (ray-finned fishes), actinistians (coelacanths and lungfishes) containing about 25,000 species. This is almost half the number of extant craniate species. The term ‘craniate’ (= with head) dates back to the times of non-evolutionist systematics, when creating a group because its members don't have what human beings have was an obvious and common way to classify the living things. In the intellectual context of fixism, the goal of the systematician was to find God’s plan in the puzzling diversity of his creatures. Many groupings defined organisms on the criterion of what they did not have, and thus classifications were full of groups for which there was no character exclusively shared by the members of the group. For example, fishes were craniates without limbs. Who has the limbs? The tetrapods, the group in which we find humans. Invertebrates are metazoans without vertebrae. Who has the vertebrae? . . . and so on.
After Darwin, the reason for biodiversity was thought to be genealogy, in other words phylogeny. Classifications were required to reflect descent of species from other species, not the God’s creation anymore. The purpose of groupings was not anymore to celebrate the perfection of humans but to demonstrate common ancestry. However, during the century between Darwin and Hennig, systematicians did not have efficient tools to fully reach this aim. They all recognized the need to abandon polyphyletic groups that include no common ancestor to all its members. But they remained in the old tradition in being unable to reject paraphyletic groups that contain a common ancestor to all its members, but this ancestor is also shared by organisms that are not included in the group. A true monophyletic group contains one ancestor and all his descendants. At that time however, both types of groups were recognized as valid. As before, paraphyletic groups were not defined for themselves, but to express a step in the increasing complexity of life, with human beings at the top. Such groups are called grades, always defining something else (complexity level, adaptation, ecology) than the organisms we put in it. The grade of reptiles would not exist as distinct from birds if one would not have the will to stress the extreme adaptation to flight in birds. Without the tetrapods, fishes would not exist and would simply be part of craniates (animals with a cranium). Without the eukaryotes, prokaryotes would not exist. What group has the nucleus in the cell? The group that includes human beings. Many other examples could be added.
With Hennig, it became possible to distinguish paraphyletic groups (containing an ancestor and only some of its descendants) from monophyletic ones (containing an ancestor and all its descendants). Hennig thus gave birth to modern systematics, where the paraphyletic groups are finally rejected. For example, the old group Pisces (‘fishes’) is clearly paraphyletic as there is no character that can exclusively define fishes. There is a common fish ancestor: it is the animal that had the first cranium, between 500 and 600 million years ago. But half of the living descendants of this ancestor are not put in ‘fishes’. These are the tetrapods. If we decided to make fishes a monophyletic group, we would have to include tetrapods, and humans would be fishes. Another way to point out paraphyly is to stress that some members of a group are more closely related to other organisms than to members of their group. For example, actinistians (coelacanths) and dipnoans are more closely related to tetrapods than to actinopterygians. Actinopterygians, as ‘bony fishes’ are more closely related to tetrapods than to chondrichthyans. The term ‘fish’ therefore disappears from modern systematics and more precise terms are now available, all related to monophyletic groups. These terms are given here only for extant taxa! Craniates have the cranium. They are made of two sister-groups, the hagfishes (mixinoids) and vertebrates, which are divided into petromyzontoids (lampreys) and gnathostomes, the jawed vertebrates. In jawed vertebrates, the chondrichthyans (defined by prismatic calcified cartilage and pelvic claspers) are the sister-group of the osteichthyans (defined by a typical pattern of dermal bones: premaxillar, maxillar, frontals, parietals, etc.). Osteichthyans are divided into two sister-groups, actinopterygians (defined by the acrodine cap on teeth and other characters) and sarcopterygians (monobasal paired fins found in lobe-finned fishes and tetrapods). Sarcopterygians contain actinistians (coelacanths) and rhipidistians defined by the sinuous aortic trunk and many other characters. Rhipidistians are made of two sister-groups, dipnoans and tetrapods.
The rise of cladistics in ichthyology starting from 1967 brought tremendous and sudden advances in systematic ichthyology. In about five years, half the teleostean tree passed from a bush to a cladogram. Today, the ‘bush at the top’ (a term due to Don Rosen and Gareth Nelson) persists, and much work remains within the terminal crown of the teleostean tree.
Guillaume Lecointre
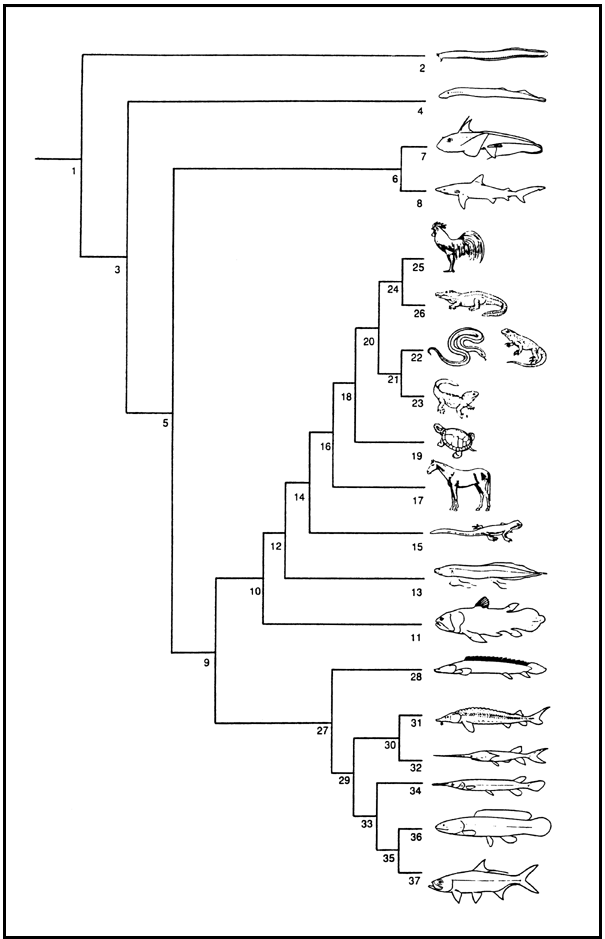
Fig. 5. A phylogeny of Craniata showing the position of the so-called "fishes" (nodes 2, 4, 6, 11, 13, 27). Node number in bold: Scientific name (Vernacular names, total number of species in the group). Note that for "fishes", species numbers are calculated from the Catalog of Fishes, Eschmeyer, Version November 2000. 1: Craniata (53,721 spp.);
2:Myxini (Myxiniformes = Hyperotreti: Hagfishes, 61 spp.); 3: Vertebrata;
4:Petromyzontiformes = Hyperoartii (Lampreys, 43 spp.); 5: Gnathostomata;
6: Chondrichthyes (907 spp.); 7: Holocephali (Chimaeras, 34 spp.); 8: Elasmobranchii (Sharks, Guitarfishes, Sawfishes, Saw sharks, Rays, Skates, Electric rays, 763 spp.);
9: Osteichthyes; 10: Sarcopterygii; 11: Actinistia (Coelacanths, 2); 12: Choanata;
13: Dipnoi (Lungfishes, 6 spp.); 14: Tetrapoda (27,541 spp.); 15: Amphibia (Lissamphibia: Frogs, Toads, Newts, Salamanders, Caecilians); 16: Amniota;
17: Synapsida (Mammalia: Mammals); 18: Sauropsida; 19: Testudines (Tortoises, Turtles); 20: Diapsida; 21 Lepidosauromorpha (Lepidosauria); 22: Squamata (Amphisbaenas, Lizards, Snakes); 23: Sphenodontida = Rhynchocephalia (Tuatara);
24: Archosauromorpha; 25: Aves (Birds); 26: Crocodylia (Alligators, Caimans, Crocodiles, Gavials); 27: Actinopterygii; 28: Cladistia (Bichirs, Reedfish, 11);
29: Actinopterygii; 30: Chondrostei; 31: Acipenseroidei (Sturgeons, 24 spp.);
32: Polyodontoidei (Paddlefishes, 2 spp.); 33: Neopterygii; 34: Ginglymodi (Gars, 7 spp.); 35: Halecostomi; 36: Halecomorpha (Bowfin, 1 sp.); 37: Teleostei (25,075 spp.).
- Name of the Order (e.g., Myxiniformes);
- Common name of the Order (e.g., Hagfishes);
- First reported occurrence in the fossil record (multiple choice fields, with Upper/Middle/Lower for both Periods and Epochs);
- Class to which the Order belongs (e.g., Myxini);
- Sister Order (e.g., Petromyzontiformes);
- Order used for Comparison (e.g., Perciformes);
- Time since shared ancestor (here: 420) million years;
- Number of Families in the Order;
- A comment field for free text description of the major features of the Order;
- A list giving access to the family(-ies) in that Order.
Status: the table is complete in that Sister Orders have been identified for all orders, as well as the times linking all Orders with shared ancestors. However, fossil discoveries and new interpretation of the fossil record will impose occasional updates of the data in this table.
You get to the ORDERS table by clicking the Order button in the FAMILIES window, or by double-clicking the Order field in the SPECIES or FAMILIES window.
On the Internet version, you get to the Orders page by clicking on the Order button in the Species Summary page.
Carroll, R. 1988. Vertebrate paleontology and evolution. W.H. Freeman, New York. 698 p.
Colbert, E. and M. Morales. 1991. Evolution of the vertebrates. John Wiley and Sons Inc., New York. 470 p.
Eschmeyer, W.N., Editor. 1998. Catalog of fishes. Spec. Publ. California Academy of Sciences, San Francisco, 3 vols. 2905 p.
Forey, P. and P. Janvier. 1993. Agnathans and the origin of jawed vertebrates. Nature 361: 129-134.
Forey, P., D. Littlewood, P. Ritchie, and A. Meyer. 1996. Interrelationships of Elopomorph fishes, p. 175-192. In M. Stiassny, L. Parenti and G. Johnson (eds.) Interrelationships of fishes. Academic Press, New York, 496 p.
Helfman, G.S., B.B. Collette and D.E. Facey. 1997. The diversity of fishes. Blackwell Science, Malden, Massachusetts. 528 p.
Nelson, J.S. 1994. Fishes of the world, 3rd ed. John Wiley and Sons, New York. 600 p.
Patterson, C. 1977. The contribution of paleontology to teleostean phylogeny, p. 579–643. In M.K. Hecht, P.C. Goody and B.M. Hecht (eds.) Major patterns in vertebrate evolution. Plenum Press, New York. 908 p.
Pough, F., J. Heiser and W. McFarland. 1989. Vertebrate life. 3rd ed. MacMillan, New York. 943 p.
Shirai, S. 1996. Phylogenetic interrelationships of Neoselachians (Chondrichthyes: Euselachii), p. 9-34. In M. Stiassny, L. Parenti and G. Johnson (eds.) Interrelationships of fishes. Academic Press, New York. 496 p.